A Global Industry Change / ENFit® ISO-80369-3
Legacy Items or All ENFit®?
What do you need? We’ll keep you connected in every way!
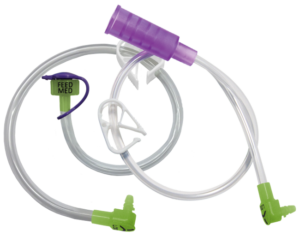
AMT Feed / Extension Sets are available in both Legacy and ENFit® configurations.
AMT’s ENFit® Options Offer:
- Forwards and Backwards Compatibility – Wide variety of ENFit® Transition Adapters!
- You can connect Current Market to ENFit®
- You can connect ENFit® to Current Market
- Glow Green Technology!
- Winged Adapters for Dexterity!
- Hybrid Feed Set Options!
- To accommodate Blenderized Diets and Venting!
- Product Packaging will include a large “Prepared for ENFit®” Sticker!
We are here to help and support you through this industry change!
AMT would like to remind our customers that we will continue to offer a complete Legacy Line and a complete ENFit® Line, pursuant to FDA regulations.
As of January 1, 2022, all GEDSA Manufacturer Members (AMT is not a member) will no longer manufacture transition sets and adaptors sold separately from other devices, as stated in the July 1, 2021, Revised ENFit® Connector Conversion Schedule.
AMT is not a member of GEDSA, and while we believe in their efforts and align with their messaging of patient safety, our position has not changed.
Part Numbers & Ordering Information
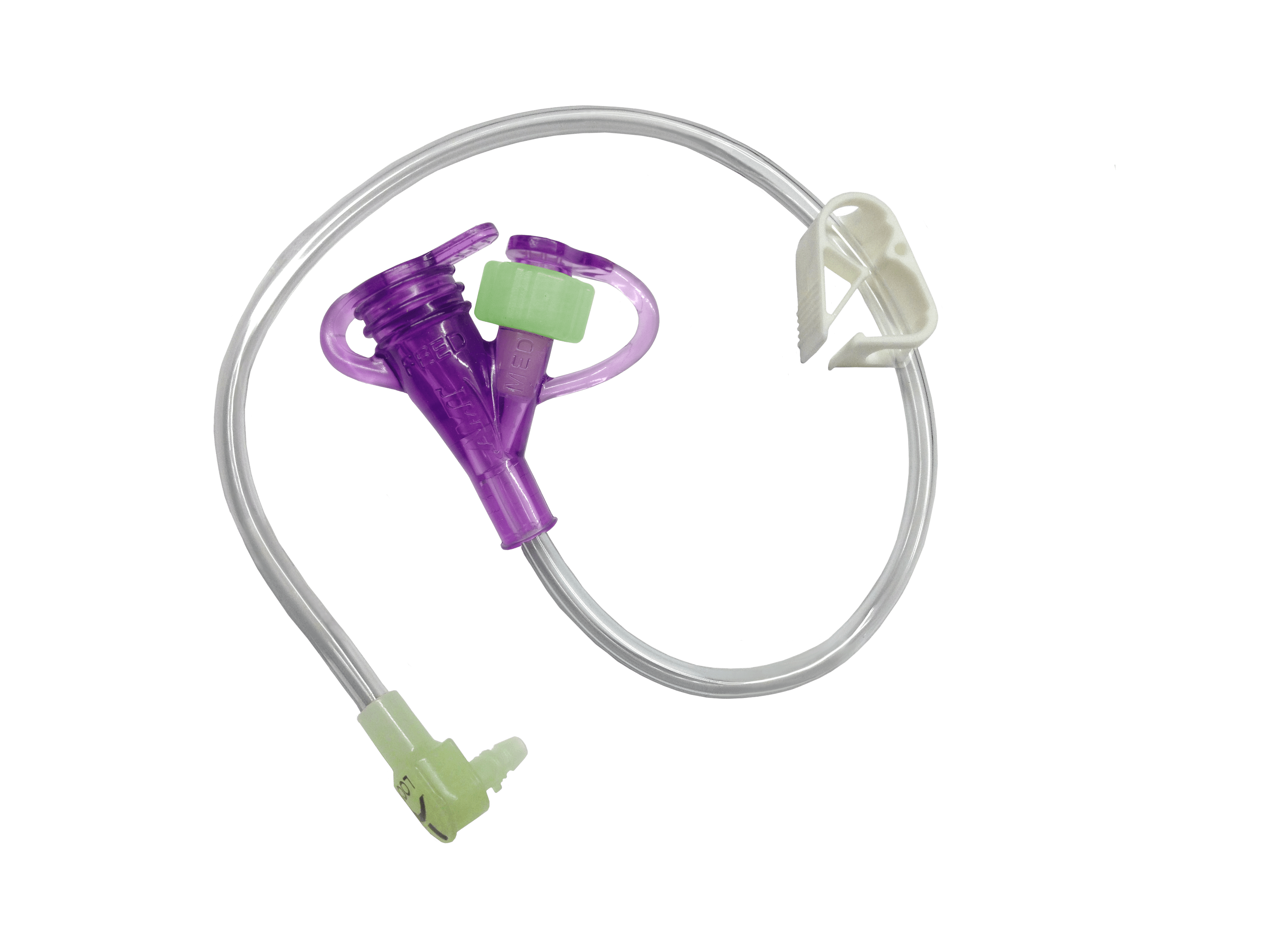
AMT Hybrid Feed Set: Legacy + ENFit®.
| Description: Hybrid Sets | Order # | Box Qty |
| 12″ Right Angle Hybrid Extension Set, Legacy + ENFit® | 8-1255-H | 10 |
| 24″ Right Angle Hybrid Extension Set, Legacy + ENFit® | 8-2455-H | 10 |

| Description: ENFit® Transition Adapters | Order # | Box Qty |
| Male ENFit® Transition Adapter | TRN101 | 10 |
| Female ENFit® Transition Adapter | TRN201 | 10 |
| ENFit® Transition Stepped Adapter | TRN102 | 10 |
| ENFit® Bolus Transition Adapter | TRN202 | 10 |
| ENFit® Y-Port Transition Adapter | TRN203 | 10 |
| ENFit® Y-Port Adapter w/ Enhanced Med-Port | TRN204 | 10 |
What is ENFit®
The International Organization for Standardization (ISO) has created a new standard design for enteral connectors, termed the “ENFit® Connector.”
These new ENFit® Connectors are intended to improve patient safety and decrease the risk of medical device misconnections. This new connector has been designed to be incompatible with Luer adapters, which are commonly used in IV applications. The ENFit® connector will look & secure very similar to a Luer threaded lock system, although the design is larger and, thus, incompatible with Luers.
Visit StayConnected.org for additional information on the ENFit® System.
Why ENFit®?
ENFit® is a patient safety initiative designed to ensure that feeding tube connectors are incompatible with the connectors for unrelated delivery systems such as trach tubes, IV lines, and catheters. Misconnections involving such medical devices may be relatively rare compared with the number of patients needing tubes or IVs, but such misconnections can have deadly consequences when they do occur.
ENFit® is a global initiative that affects end users, manufacturers, and suppliers. The ENFit® Connectors were developed as part of the Stay Connected initiative under the supervision of clinicians, manufacturers, and regulators.

What is AMT’s Role?
AMT will comply with the enteral connector requirements of ISO 80369-3, better known as ENFit®, established by the governing bodies to manage the changeover. During the interim period, AMT has made enteral feeding transition adapters available to smooth the transition while continuing to protect patients.
What Does this Mean for You?
What is changing is how you connect your food source to the feed set, which in turn connects to an enteral feeding device. The straight or right angle connectors, which attach and lock into low-profile feeding devices, will remain the same. The distal end of feed sets, which connect directly into the device, are not regulated at this time. However, the proximal side of the feed set, your bolus or y-port adapters, will begin to incorporate the ENFit® design into newly created product lines.
Understanding ENFit®
From where we are today, to where you’re going tomorrow!
Current Enteral Administration
The current administration sets to enteral feeding devices consist of: (1) Catheter Tip Syringes, (2) Stepped/Christmas Tree Adapters, (3) Luer Locking Syringes, (4) Luer Slip Syringes & (5) Oral Syringes.
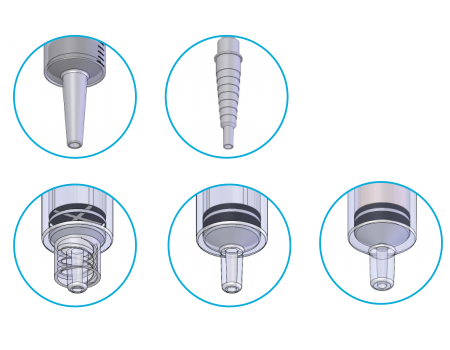
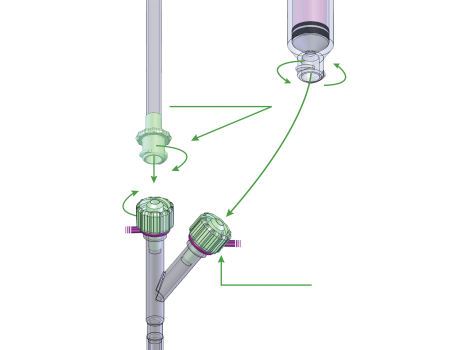
ENFit® Transition Adapters
ENFit® male transitional adapters will be available to attach to the new administration sets with the ENFit® female connectors. This will facilitate compatibility between the new ENFit® system and the current existing part system as the market transitions.
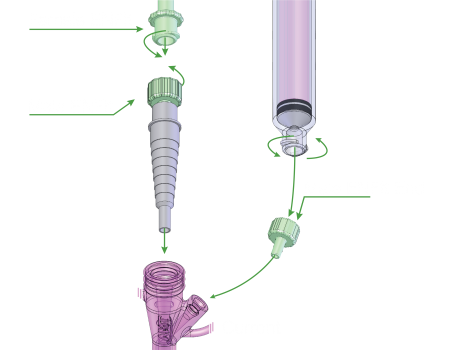
Transition to Standard
The new ENFit® system has been designed to reduce the misconnections between unrelated delivery systems (i.e. vascular, respiratory, epidural). Both enteral administrative sets and device sets will adjust to the ENFit® standard.
ENFit® Frequently Asked Questions
AMT has provided this information as an educational resource tool. This is not intended as a substitute for professional medical care.
Your FIRST source of information should be your healthcare provider.
WHAT IS THE ENFIT® CONNECTOR ISO 80369-3 CHANGE?
In order to improve patient safety and to decrease the risk of medical device misconnections, the International Organization for Standardization (ISO) has created a new standard design for enteral connectors, termed the “ENFit® Connector.” This new connector has been designed to be incompatible with Luer adapters, which are commonly used in IV applications. The ENFit® connector will look & secure very similar to a Luer threaded lock system, although the design is larger and, thus, incompatible with Luers.
WHAT IS THE PLANNED ROLLOUT FOR THE ENFIT® CONNECTOR CHANGE?
Feeding bag manufacturers have started to make the giving set (food source) end with a female ENFit lock (instead of the current Christmas tree adapter). Some syringe manufacturers have already begun making syringes specifically for enteral use, having tips with a female ENFit lock just like the feeding bag giving set (instead of the current catheter or luer tips typical of bolus syringes).
Once both the food source ends (bag and syringe) have been implemented, the feeding set (device) manufacturers will start making feeding sets that have a male ENFit lock (instead of the traditional bolus or Y-port). Note, the end of the feeding (device) sets that connect to the device (straight & right angle adapters) will not change and will still be manufacturer-specific (e.g. MiniONE® compatible).
During the changeover for each of the components above, transitional adapters will be made available by both the feeding bag and feeding device manufacturers that allow for backward compatibility with older connectors.
HOW IS AMT PLANNING THE ROLLOUT OF THIS NEW PRODUCT?
AMT will remain in compliance with all rules and regulations established through ISO 80369-3 and any applicable FDA regulations regarding the time frame release of the ENFit® connectors.
AMT ENFit® transition adapters are currently available for forward and backward compatibility.
Additionally, AMT offers both a complete ENFit® line and Legacy line of products to accommodate our customers’ needs.
WILL THERE BE SAMPLES AVAILABLE FOR PRODUCT DEMONSTRATIONS?
Yes. AMT’s sales team will have product samples to perform in-servicing opportunities. We will also provide additional training tools as the new ENFit® connectors come to market.
WHAT IS THE TIMING OF THE NEW ENFIT® ENTERAL CONNECTOR TRANSITION?
Visit StayConnected.org for additional information and current timelines on the ENFit® System.
WHEN WILL THE TRANSITION PIECES BE AVAILABLE?
AMT ENFit® transition adapters are already available. AMT also currently offers a full line of ENFit®-compatible feeding extension sets. The transition pieces allow the use of both the current feeding tube connectors and the new ENFit® connectors.
HOW LONG WILL THE TRANSITION PIECE BE AVAILABLE?
AMT will offer transitional adapters as consumers’ needs adjust. The FDA may eventually require the transitional adapters be discontinued, such that full ENFit® integration occurs.
WILL THE TRANSITION PIECE BE SOLD AS A SEPARATE ITEM?
Yes.
ENFit® is a registered trademark of GEDSA, Inc. or its affiliates.
